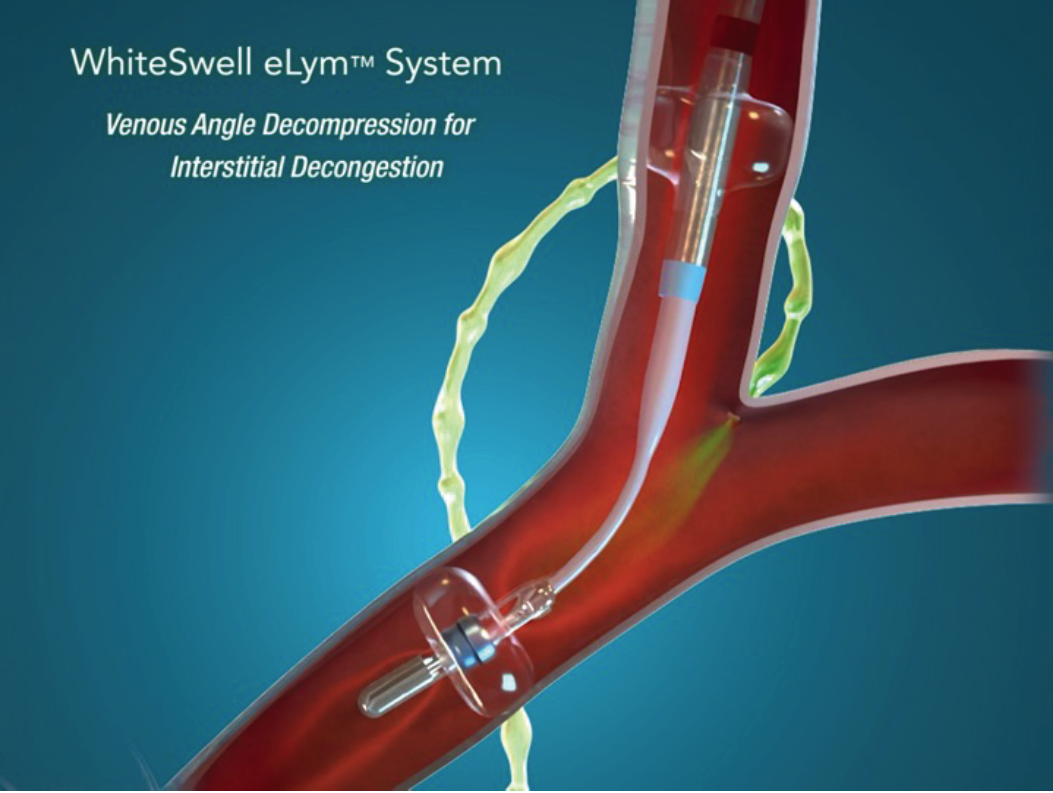
Leg swelling and difficulty breathing in acute decompensated heart failure (ADHF) are common symptoms that indicate excess fluid in the tissues and organs. Current therapy targets vascular congestion but does not directly target tissue and organ congestion. Even after intravenous diuretic therapy, residual congestion can persist, contributing to poor outcomes. 1, 2, 3
A critical link is the ability of the lymphatic system to remove fluid from the tissue and organs to maintain fluid balance. Lymphatic vessels throughout the body drain and filter 8 liters of fluid per day out of interstitial tissues to the vascular system, where it can be removed with diuretic therapy. In ADHF, the lymphatic system is overwhelmed and cannot effectively drain fluid, causing edema. 4, 5, 6